This post was contributed by Mr. Tuggy’s Chemistry class! Mr. Tuggey has been teaching IB chemistry at ISAK since 2015. Known for his theatrical teaching style in the classroom, Mr. Tuggey also enjoys challenging students to outdoor adventures including climbing, hiking and caving.
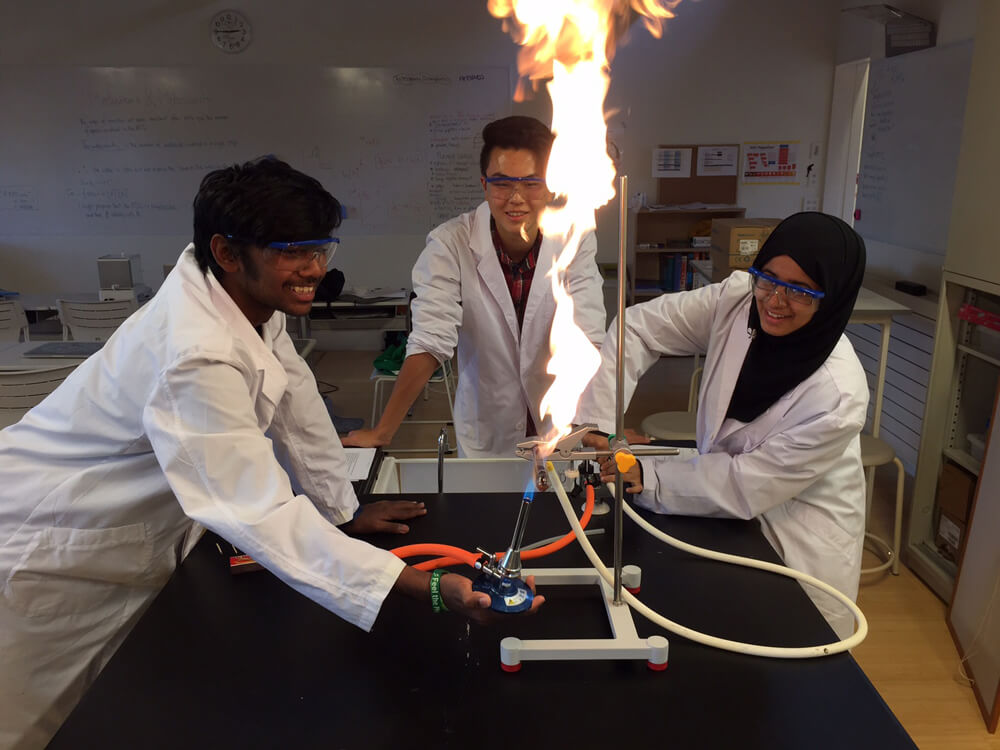 IB Chemistry covers some pretty challenging topics, including atomic theory, periodicity, chemical bonding and structure, equilibrium, acids and bases, energetics, thermodynamics, redox processes and more. Mr. Tuggey, enjoys designing experiments that bring key concepts to life. In a second term experiment, students were tasked with determining the formula copper oxide.
IB Chemistry covers some pretty challenging topics, including atomic theory, periodicity, chemical bonding and structure, equilibrium, acids and bases, energetics, thermodynamics, redox processes and more. Mr. Tuggey, enjoys designing experiments that bring key concepts to life. In a second term experiment, students were tasked with determining the formula copper oxide.
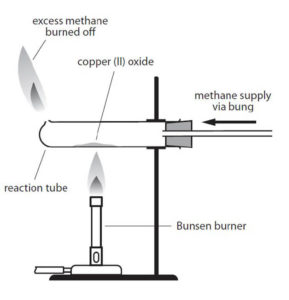 Copper oxide (CuO), also known as cupric oxide or tenorite, is a product of the copper mining industry. Working in teams of two, students heated copper(II) oxide in a glass tube while at the same time passing methane over it. As the copper oxide was reduced to copper, the reactants and products could then be weighed so that the formula for copper oxide could accurately be calculated.
Copper oxide (CuO), also known as cupric oxide or tenorite, is a product of the copper mining industry. Working in teams of two, students heated copper(II) oxide in a glass tube while at the same time passing methane over it. As the copper oxide was reduced to copper, the reactants and products could then be weighed so that the formula for copper oxide could accurately be calculated.
The giant flames created as the excess methane burned off were quite exciting!!
Here is an example of a solution (shared from rsc.org):




